Many of us have been brought up with the following chart, which represents how nutrient availability to plants varies with the pH level, and we use it a “proof” of the need for proper pH control in our fertilizer solutions.
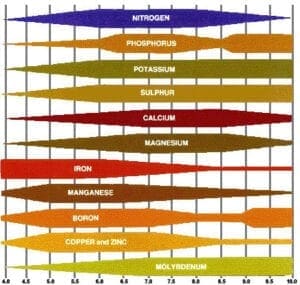
While nobody can argue that proper pH plays a significant role in plant culture, this chart is something that might fit in the “urban legend” category. It may be a real representation of data collected in a study, but is actually of little value to orchid growers, as it was derived from a single study, using a single formula of fertilizer, using a single SOIL substrate.
The fact that it is from a single study makes it somewhat suspect to begin with.
Not all minerals dissociate the same in aqueous solutions, and that can also be affected differently by pH. Do we know what minerals were in the tested fertilizer, their relative ratios and concentrations, and how they compare to what we use?
The fact that it is pour-through results from a soil substrate really confounds its value to orchid growers, as soils can have significant cation exchange capacities (CEC), while most orchid media have orders-of-magnitude less, if at all.
The cation exchange capacity is a measure of how well soil components hold onto positively-charged ions – cations – and most of the CEC occurs at the edges of clay particles*. The development of positive and negative charges on a clay particle is affected strongly by pH, so we can understand how, at extreme pH values, the charges may strongly bind the cations in fertilizer solutions, making them unavailable to the plants. In orchid media, which tend to have essentially no CEC, the nutrient cations tend to stay in solution and remain available.
* This does not pertain to the clay in LECA, as they are fired clay bodies, and are therefore sintered and to some extent vitrified, which ties up the site of charge development. LECA has almost no CEC
